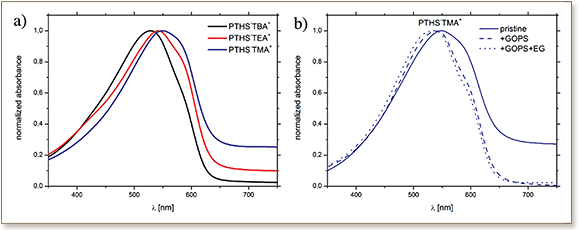
Conjugated polyelectrolytes (CPEs) are a focus of research because combine their inherent electrical conductivity and the ability to interact with ions in aqueous solutions or biological systems. However, it is still not understood to what degree the counter ion in CPEs influences the properties of the CPE itself and the performance of electronic transducers. In order to investigate this, three different conjugated polyelectrolytes, poly(6-(thiophen-3-yl)hexane-1-sulfonate)s (PTHS−X+), are synthesized, which have the same polythiophene backbone but different X+counter ions: the bulky tetrabutylammonium (TBA+), tetraethylammonium (TEA+), and the smallest tetramethylammonium (TMA+). At the interface with biological systems, thin CPE films have to be stable in an aqueous environment and should allow the inward and outward flow of ions from the electrolyte. Since the studied PTHS−X+ have different solubilities in water, the optical properties of pristine PTHS−X+ as well as of crosslinked PTHS−X+ via UV–vis absorption spectroscopy are investigated additionally. PTHS−TMA+ exhibits better aggregation, fast interdiffusion of ions, and fast recovery from the oxidized state. Additionally, spectroelectrochemical and cyclic voltammetric as well as electrochemical capacitance investigations show that PTHS−TMA+ can be oxidized to a higher degree. This leads to a better performance of PTHS−TMA+-based organic electrochemical transistors.
