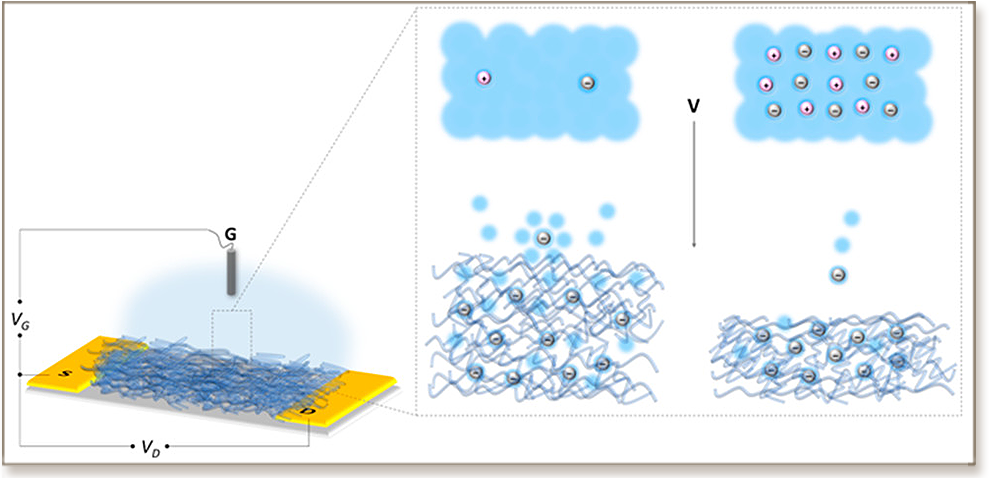
Organic electrochemical transistors (OECTs) composed of organic mixed conductors can operate in aqueous, biological media and translate low-magnitude ionic fluctuations of biological origin into measurable electrical signals. The growing technological interest in these biotransducers makes the fundamental understanding of ion-to-electron coupling extremely important for the design of new materials and devices. One crucial aspect in this process that has been so far disregarded is the water taken up by the film during device operation and its effects on device performance. Here, using a series of the same electrolyte with varying ion concentrations, we quantify the amount of water that is incorporated into a hydrophilic p-type organic semiconductor film alongside the dopant anions and investigate structural and morphological changes occurring in the film upon electrochemical doping. We show that infiltration of the hydrated dopant ions into the film irreversibly changes the polymer structure and negatively impacts the efficiency, reversibility, and speed of charge generation. When less water is injected into the channel, OECTs exhibit higher transconductance and faster switching speeds. Although swelling is commonly suggested to be a necessity for efficient ion-to-electron transduction, this work uncovers the negative impact of a swollen channel material on the performance of accumulation mode OECTs and lays the foundation for future materials design.