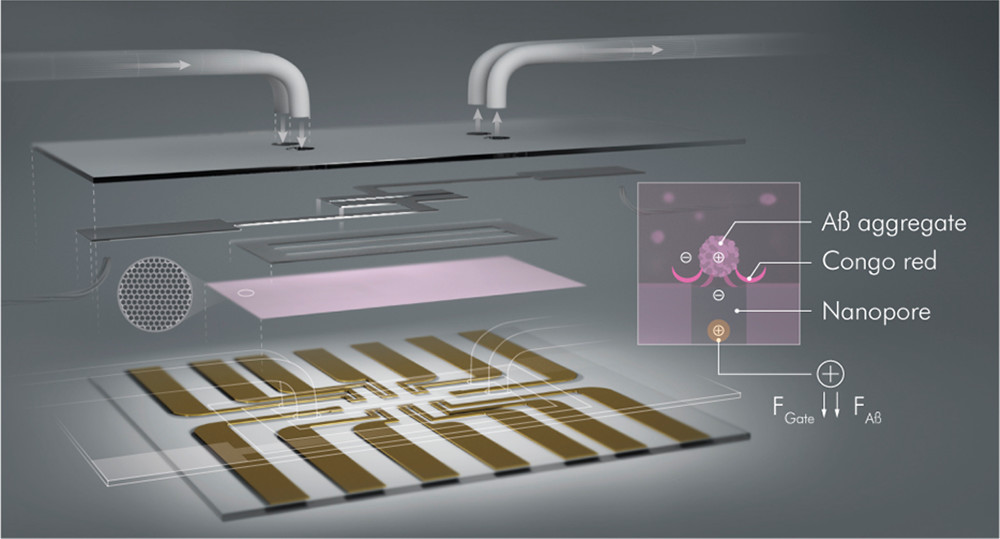
Alzheimer’s disease (AD) is a neurodegenerative disorder associated with a severe loss in thinking, learning, and memory functions of the brain. To date, no specific treatment has been proven to cure AD, with the early diagnosis being vital for mitigating symptoms. A common pathological change found in AD-affected brains is the accumulation of a protein named amyloid-β (Aβ) into plaques. In this work, we developed a micron-scale organic electrochemical transistor (OECT) integrated with a microfluidic platform for the label-free detection of Aβ aggregates in human serum. The OECT channel–electrolyte interface was covered with a nanoporous membrane functionalized with Congo red (CR) molecules showing a strong affinity for Aβ aggregates. Each aggregate binding to the CR-membrane modulated the vertical ion flow toward the channel, changing the transistor characteristics. Thus, the device performance was not limited by the solution ionic strength nor did it rely on Faradaic reactions or conformational changes of bioreceptors. The high transconductance of the OECT, the precise porosity of the membrane, and the compactness endowed by the microfluidic enabled the Aβ aggregate detection over eight orders of magnitude wide concentration range (femtomolar–nanomolar) in 1 μL of human serum samples. We expanded the operation modes of our transistors using different channel materials and found that the accumulation-mode OECTs displayed the lowest power consumption and highest sensitivities. Ultimately, these robust, low-power, sensitive, and miniaturized microfluidic sensors helped to develop point-of-care tools for the early diagnosis of AD.